

Client: UK Government
Developing an emergency ventilator in 6 weeks
Answering the UK Government's call to deliver safety critical ventilators.
In March 2020, the rising COVID-19 pandemic posed an unprecedented threat to the UK health system, with the potential risk of ventilator shortages. While medical device development often takes years, we were asked to develop an emergency ventilator in just weeks.
Working closely with a UK manufacturer, we adapted and scaled their existing technology to meet the national requirements for addressing COVID-19. Using an Agile, systems engineering approach, our team worked on multiple activities at the same time, including mechanical design, pneumatics and hydraulics, software and electronics, industrial design, human factors, risk management, testing, quality assurance, transfer to manufacture and technical file generation.
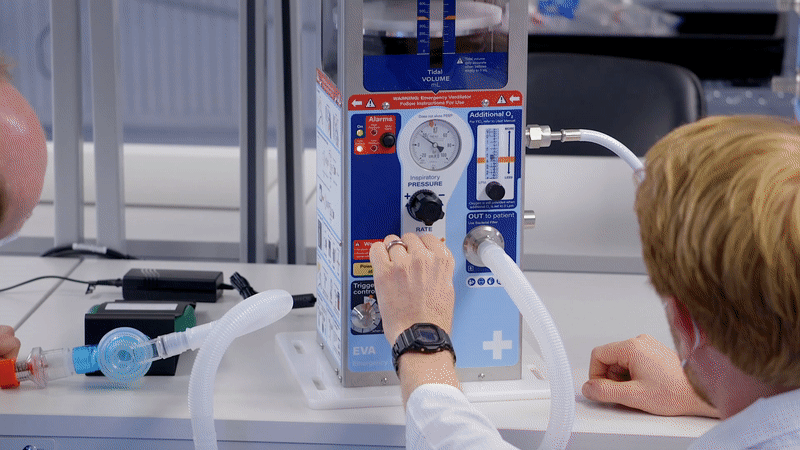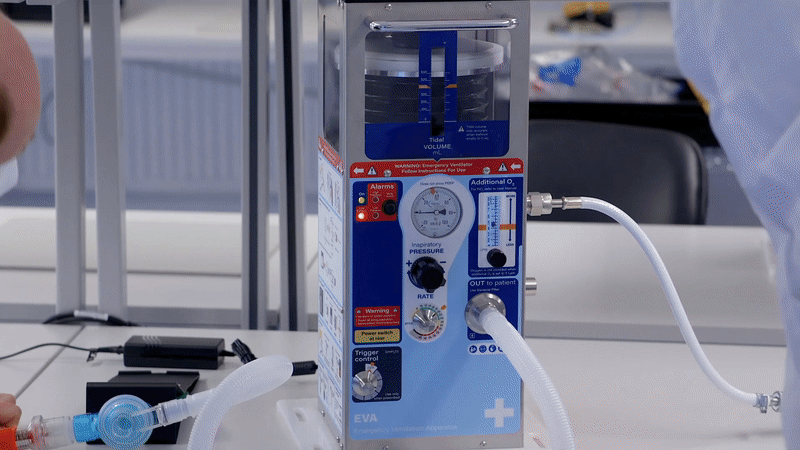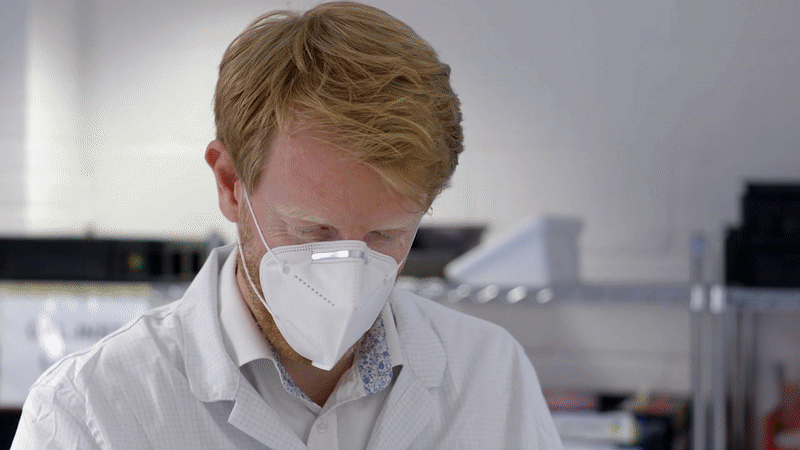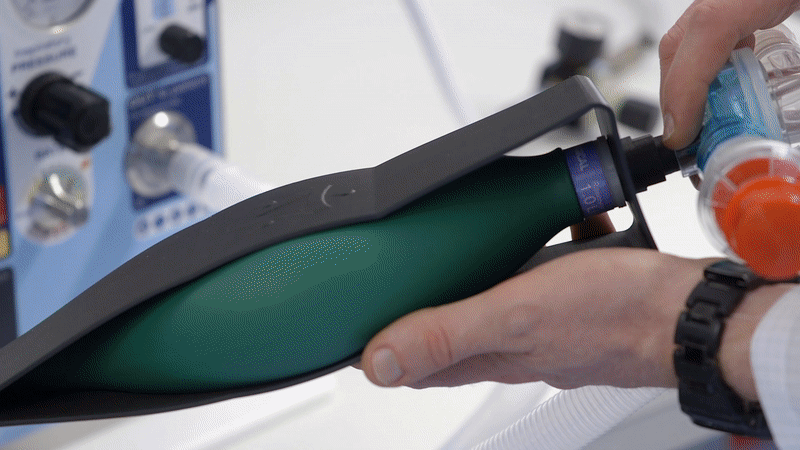
This was an immense effort, leading to the Emergency Ventilator Apparatus (EVA 1.5) being developed with a design freeze in 4 weeks, before being transferred to manufacture in 6 weeks.







At Team, we are 100% focused on medical device development and know that no safety critical device has been developed from scratch to CE mark in only a few weeks. To allow the fastest possible delivery and avoid supply chain risk, we based our design on an existing UK ventilator that had been proven to save lives under emergency conditions. Recognising that many organisations were at that point seeking to source sophisticated ICU ventilators, our approach was to design a ventilator that could function under worst-case lockdown conditions.
Steve Blatcher, EVA Product Manager.






User feedback is essential in any medical device development. With the first lockdown upon us, we had to quickly implement an effective way to get feedback from clinicians who had experience with COVID-19 patients and ventilators. Thanks to these early discussions, we learnt a lot about how things were done and used this knowledge to make EVA as usable as possible.
Derek Dumolo, Human Factors Consultant.






To make the device both user friendly and accessible in emergency conditions, we focused on producing clear and simple set-up instructions which we integrated into the bodywork of EVA. The device needed to be used by both clinical staff and potentially by non-critical staff too, with training, so it was important to achieve simple, clear instructions for use.
Jess Fox, Human Factors Consultant.






Designed and developed by Team Consulting for the UK Government
We are an award-winning medical device consultancy, working with companies to design and develop medical devices. That’s all we do and we are proud of this focus.
Could we help with your next project?
